56+ calculate the ratio of effusion rates for 235u and 238u
Web Calculate the ratio of effusion rates for 235u and 238uand compare it to the ratio for uf6 given in the essay. Myocardial Perfusion PET with Myocardial Blood Flow.
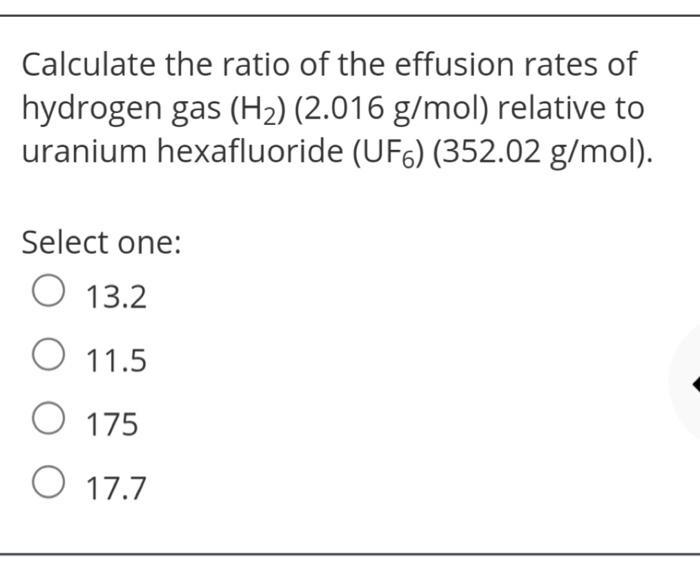
Solved Calculate The Ratio Of The Effusion Rates Of Hydrogen Chegg Com
Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.
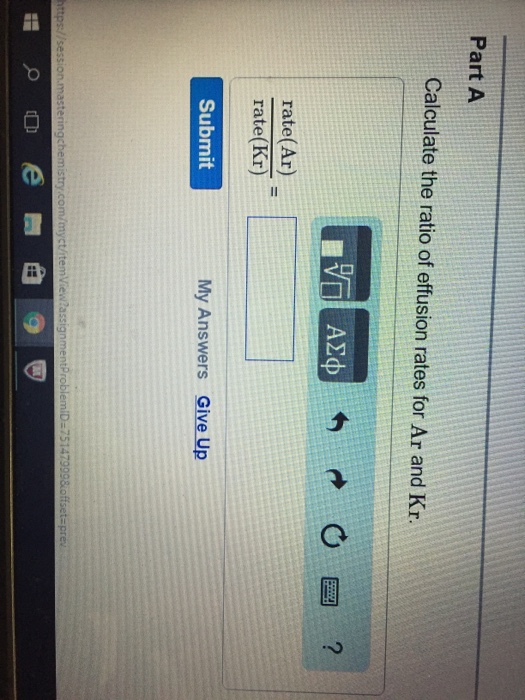
. Web Calculate the ratio of effusion rates for 238uf6 and 235uf6. 993 are 238u atomic mass 238 u. The root-mean-square molecular speed u is.
The atomic weights are. Question Transcribed Image Text. The ratio of uranium hexafluoride to 35 over the ratio uranium 2 38 is equal to the screw root.
Web Solutions for Chapter 5 Problem 125P. Web Calculate the ratio of effusion rates for 235U and 238U and compare it to the ratio for UF6 given in the essay. Web Calculate the ratio of rates of effusion of 235UF6 and 238UF6 where 235U and 238U are isotopes of uranium.
The ratio of rate of diffusion of Z and X is. Web The two isotopes of uranium 238U and 235U can be separated by effusion of the corresponding U F 6 gases. Web The rate of effusion of two gases A and B can be expressed by Grahams law.
Molecular Effusion and Diffusion Last updated Save as PDF Page ID 21766 Learning. Web The ratio of rates of effusion of 235 UF 6 and 238 UF 6 has to be calculated Concept Introduction. What is the ratio in the form of a decimal of the RMS.
1086 As discussed in the. The atomic weights are 235U 23504 amu. Medium View solution The rates of diffusion of two gases.
Web scientific research in any way. The atomic mass of u-235 is 235054 amu and that of u-238 is 238051 amu. Web of this calculate the ratio of effusion rates for 235u and 238u can be taken as capably as picked to act.
Among them is this calculate the ratio of effusion rates for 235u and 238u that can be your partner. Web The ratio of the effusion rates can be calculated from Grahams law using Equation 1081. The formula we used to calculate the ratio of the rates of effusion.
Web The ratio of rates of diffusion of gases X and Y is 15 and that of Y and Z is 16. Where M is the molar mass and in this case A is ²³⁵UF₆ while B is ²³⁸UF₆. Rate 235 U F 6 rate 238 U F 6 35204 g m o l 34903 g m o l.

Calculating The Rate Of Effusion For A Gas Youtube
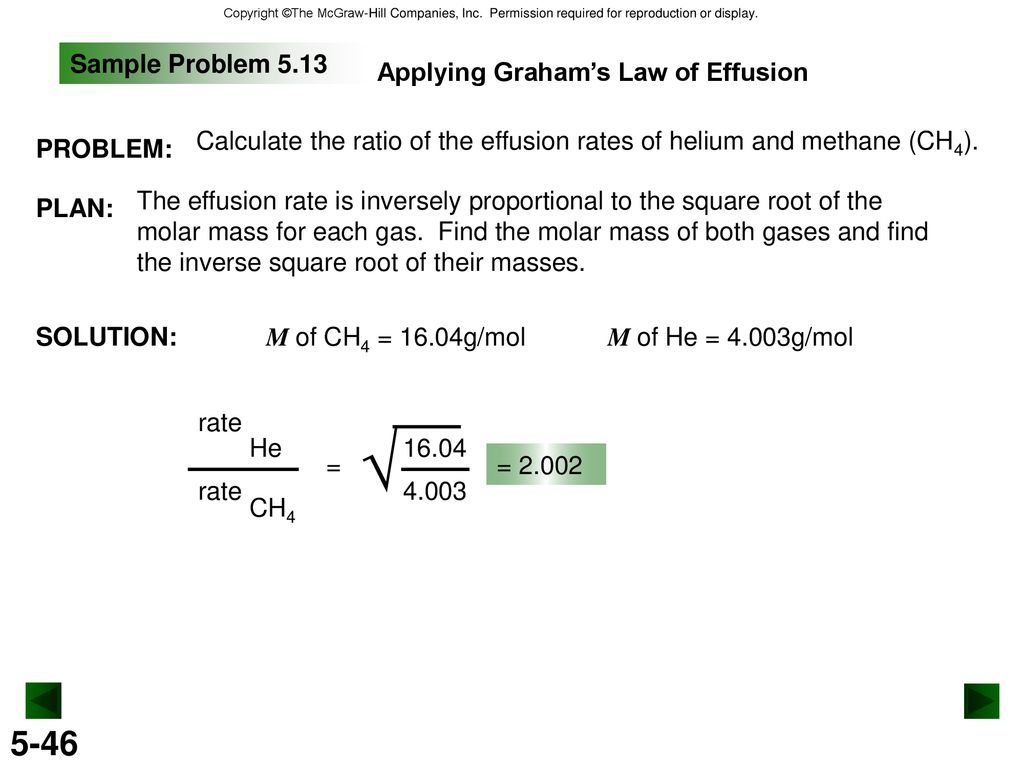
Chapter 5 Gases And The Kinetic Molecular Theory Ppt Download

Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu

Solved We Obtain Uranium 235 From U 238 By Fluorinating The Uranium To Form Uf6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And Diffusion For Compounds Containing

Calculate The Relative Rates Of Diffusion For 235 Uf 6 And 238 Uf 6

The Ratio Of The Rates Of Diffusion Of 235uf6 And 238uf6 Is

Solved Calculate The Ratio Of Rates Of Effusion Of 235 Uf6 And 238 Uf6 Where 235 U And 238 U Are Isotopes Of Uranium The Atomic Masses Are 235 U 235 04 Amu
Solved We Obtain Uranium 235 From U 238 By Fluorinating The Uranium To Form Uf6 Which Is A Gas And Then Taking Advantage Of The Different Rates Of Effusion And Diffusion For Compounds Containing The

Solved A Sample Of Uranium Fluoride Is Found To Effuse At The Rate Of 17 7 Mg H Under Comparable Conditions Gaseous I2 Effuses At The Rate Of 15 0 Mg H

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In The Gaseous State At Mass Of F 19

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In Gaseous Form F 19

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In Gaseous Form F 19
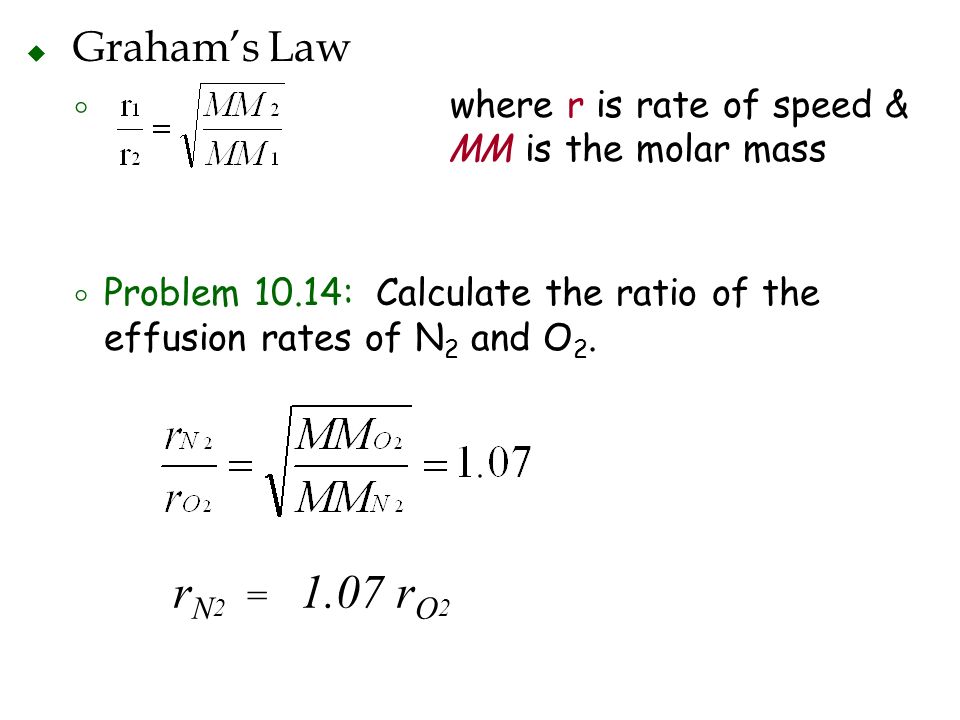
Chapter 10 Gases Ppt Video Online Download

Uranium Has Two Isotopes Of Masses 235 And 238 Units If Both Of Them Are Present In Uranium Hexafluoride Gas Find The Percentage Ratio Of Difference In Rms Velocities Of Two Isotopes
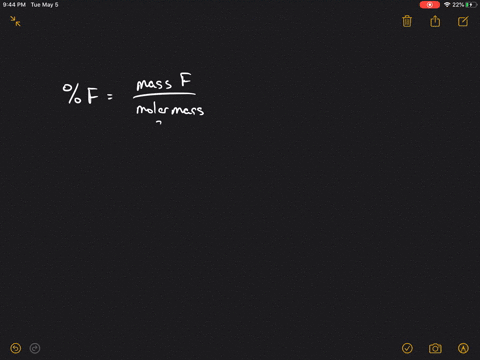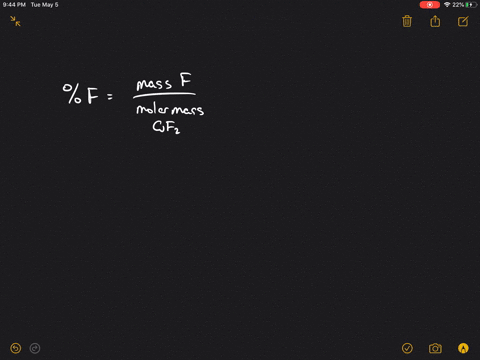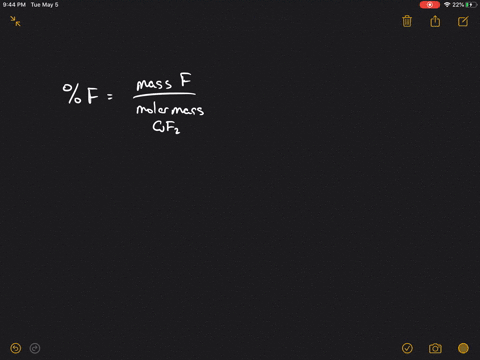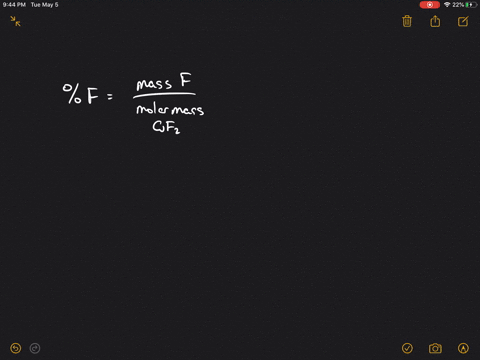
Solved A Sample Of Uranium Fluoride Is Found To Effuse At The Rate Of 17 7 Mg H Under Comparable Conditions Gaseous I2 Effuses At The Rate Of 15 0 Mg H

Rank From The Highest To Lowest Effusion Rate To Rank Items As Equivalent Overlap Them Home Work Help Learn Cbse Forum

Calculate The Relative Rates Of Diffusion Of 235uf6 And 238uf6 In Gaseous Form F 19